Case Study: Pulmonary Deposition
• Gamma scintigraphy study to determine pulmonary deposition from a novel fixed dose triple combination (ICS, LAMA,LABA) pressurised metered dose inhaler (pMDI)
• Two period cross-over study to determine the effect of breath-hold (10 s vs 3 s) on deposition parameters
Radiolabelling Objective
• Critical to develop and validate a method for incorporation of a suitable radiolabel i.e. Technetium-99m (99mTc) into the pMDI whereby it behaves as an accurate surrogate for the active pharmaceutical ingredients (APIs).
• Furthermore the delivered dose and aerodynamic particle size distribution (APSD) of the formulation must not be changed by the addition of 99mTc
• The principles for demonstrating appropriate radiolabeling documented in the standardised techniques developed by the International Society for Aerosols in Medicine (ISAM) Regulatory Affairs Networking Group were adhered to (Devadason et al., 2012).
Radiolabelling Validation
• Radiolabelled batches were compared to non-radiolabelled batches to ensure that the radiolabeling process did not alter the delivered dose of the APIs.
• Standard pharmacopoeial inertial impaction tests were performed using the Next Generation Impactor (NGI) to demonstrate the correlation between the deposited drugs and radioisotope.
• API deposition was determined by validated HPLC-UV. Radiolabel distribution was determined using the gamma camera.
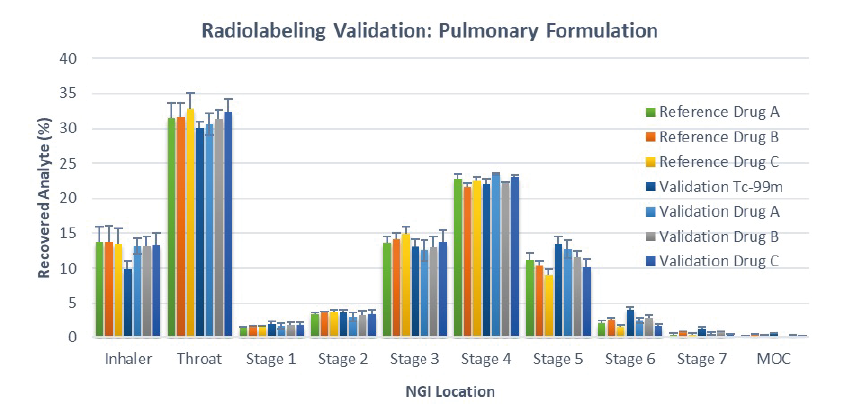
• Figure 1 shows the NGI distribution patterns of reference drug i.e. standard commercial pMDI (n = 8) and drug and 99mTc deposited from the radiolabelled validation batches (n = 5)
Figure 1: APSD of the % recovered analytes i.e. Drug A, B & C and 99mTc from radiolabelled pMDI and the corresponding reference product (reference batch, n= 8; validation batch, n=5)
Quality Control Tests: Clinical Phase
• Radiolabelled canisters were manufactured, on each dosing
day, according to approved Master Batch Records and in
compliance with GMP.
• Prior to product release the delivered dose (MBq of 99mTc)
and the APSD of the APIs and the 99mTc were determined, to
ensure they conformed with release criteria.
• In total 11 batches were produced during the clinical phase
and all batches complied with the release specifications.
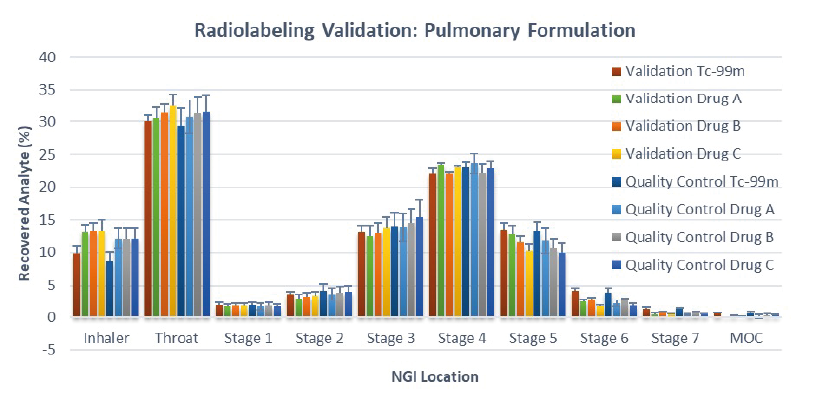
Figure 2: APSD of the % recovered analytes i.e. Drug A, B & C and 99mTc from validation and clinical radiolabelled products (validation batch, n=5; quality control batch, n=11)
Clinical Study
This scintigraphy study was the first to evaluate the lung deposition of a novel fixed-dose triple combination pMDI using co-suspension technology (Israel et al., 2020).
During the study, each healthy volunteer underwent a 81mKr gas ventilation scan to define the lung margins (Figure 3a) and a 57Co transmission scan to determine regional tissue attenuation (Figure 3b).
On the dosing day, subjects were administered a single dose i.e. two inhalations from the radiolabelled pMDI, followed by a 10 or 3 second breath hold depending on randomisation.
Simultaneous anterior and posterior views of the lungs and stomach, and head/neck were performed immediately post dose, and regional lung deposition i.e. inner (I) and outer (O) regions of interest (ROI), were assessed. Figure 3a shows the I & O regions superimposed over the ventilation image.
The study was conducted in compliance with the standardisation guidance published by ISAM (Newman et al., 2012).
Results
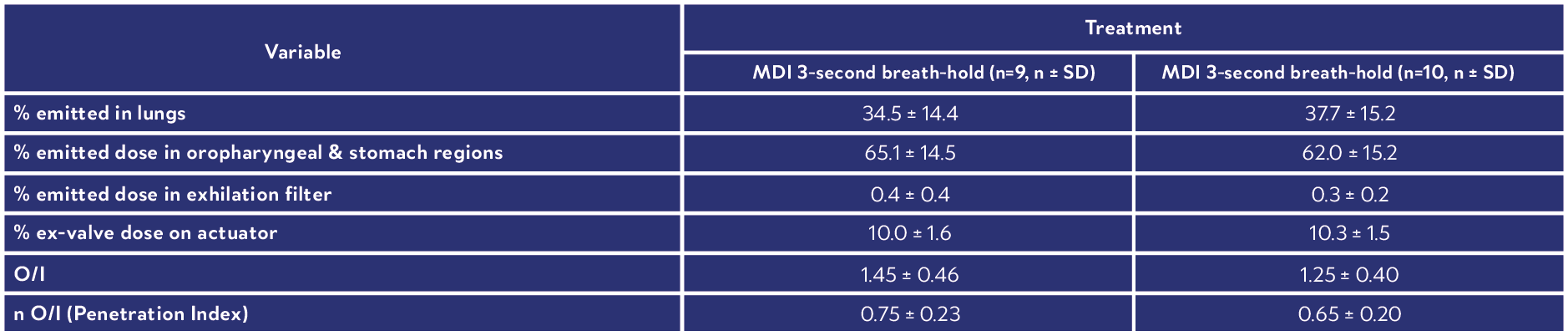
This novel fixed dose triple combination (ICS, LAMA, LABA) pMDI consistently delivered a high lung dose (see Table 1).
Results showed similar regional depositions for each breath hold manoeuvre (Figure 4), suggesting the length of breath-hold had no significant effect on total lung and peripheral deposition patterns of this novel co-suspension delivery system (Table 1).
It was concluded that this product is suitable for use by asthma and COPD patients who may not be able to perform long breath holds.
Figure 3: (a) 81mKr gas ventilation, (b) 57Co transmission image.
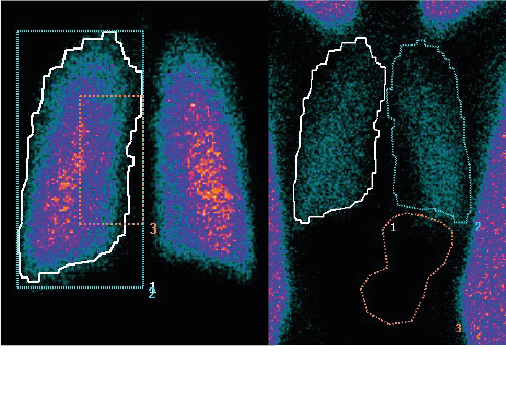
Figure 4: (a) Deposition following 10-second breath-hold and (b) 3-second breath-hold
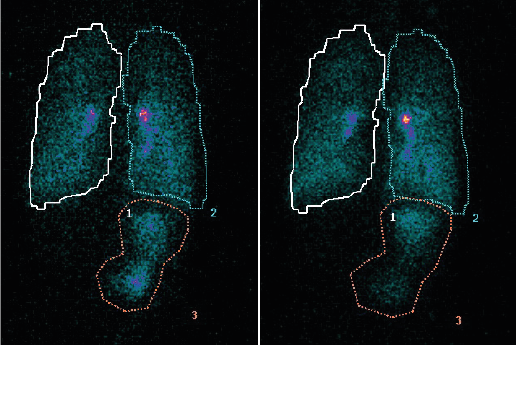
Images shown are from the same volunteer. Figures 3 a, 4 a & b show reflected posterior views, Figure 3 b is the anterior view.
Table 1: Summary of derived deposition data
Conclusions
• Effective radiolabeling validation demonstrated that the 99mTc radiolabel was an accurate surrogate for the APIs.
• Daily quality control tests of the radiolabelled products confirmed that the pMDIs used for clinical dosing complied with rigorous release specifications.
• Accurate determination of the in vivo deposition of APIs from a novel fixed dose combination pMDI was achieved in a group of healthy volunteers.
References
• Devadason et al. (2012), Validation of radiolabelling of drug formulations for aerosol deposition assessment of orally inhaled products, Journal of Aerosol Medicine and Pulmonary Drug Delivery, 25(S1): S6-S9. https://doi.org/10.1089/jamp.2012.1Su3
• Israel et al. (2020), Pulmonary deposition of budesonide/glycopyrronium/formoterol fumarate dehydrate metered dose inhaler formulated using co-suspension delivery technology in healthy male subjects, European Journal of Pharmaceutical Sciences, 153: 10572. https://doi.org/10.1016/j.ejps.2020.105472
• Newman et al. (2012) Standardization of Techniques for Using Planar (2D) Imaging for Aerosol Deposition Assessment of Orally Inhaled Products, Journal of Aerosol Medicine and Pulmonary Drug Delivery, 25 (S1): S10-S28. https://doi.org/10.1089/jamp.2012.1Su4
